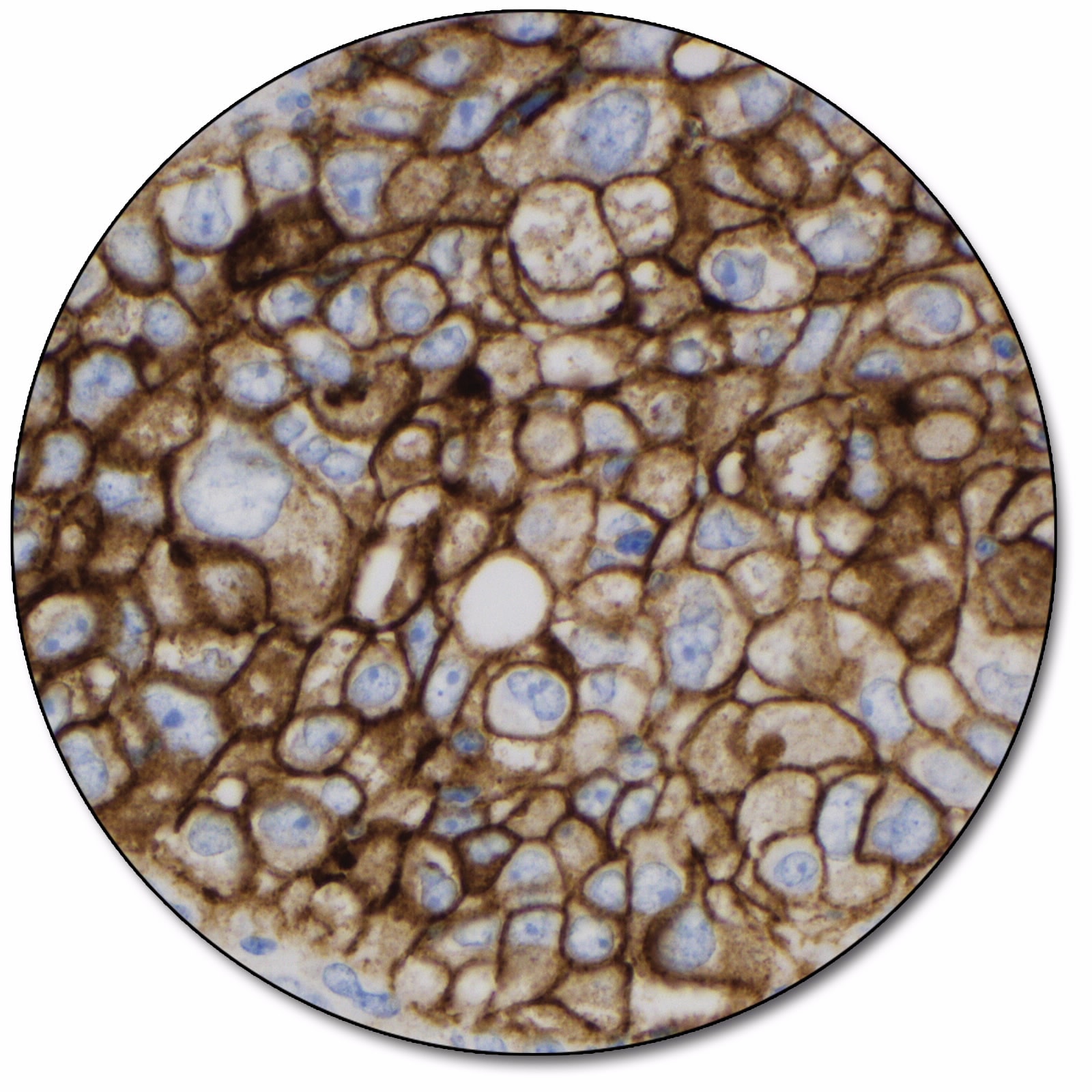
Non-small cell lung cancer
PD-L1 IHC 22C3 pharmDx for Autostainer Link 48
IVD

PD-L1 IHC 22C3 pharmDx is a qualitative immunohistochemical assay using Monoclonal Mouse Anti-PD-L1, Clone 22C3 intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) non-small cell lung cancer (NSCLC), esophageal squamous cell carcinoma (ESCC), cervical cancer, head and neck squamous cell carcinoma (HNSCC), triple-negative breast cancer (TNBC), and gastric or gastroesophageal junction (GEJ) tissues using EnVision FLEX visualization system on Autostainer Link 48.
PD-L1 protein expression in NSCLC is determined by using Tumor Proportion Score (TPS), which is the percentage of viable tumor cells showing partial or complete membrane staining at any intensity.
PD-L1 protein expression in ESCC, cervical cancer, HNSCC, TNBC, and gastric or GEJ adenocarcinoma is determined by using Combined Positive Score (CPS), which is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100.
PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying NSCLC, ESCC, cervical cancer, HNSCC, TNBC, and gastric or GEJ adenocarcinoma patients for treatment with KEYTRUDA? (pembrolizumab). PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying NSCLC patients for treatment with LIBTAYO? (cemiplimab-rwlc). Please refer to the full intended use for indications and PD-L1 expression levels.
For details on staining interpretation, refer to section 13 of the Instructions for Use and indication-specific PD-L1 IHC 22C3 pharmDx Interpretation Manuals.
PD-L1 IHC 22C3 pharmDx
The kit includes reagents required for the immunohistochemical staining (except wash buffer), control slides representing positive and negative PD-L1 protein expression, and detailed instructions. The kit has been tailored especially for use on Autostainer Link 48 instruments. The materials provided are sufficient for 50 tests (50 slides incubated with monoclonal mouse antibody to PD-L1 and 50 slides incubated with the corresponding negative control reagent, 100 slides in total).
For countries outside of the United States, see the local KEYTRUDA product label for approved indications and expression cutoff values to guide therapy.
KEYTRUDA? is a registered trademark of of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
LIBTAYO is a registered trademark of Regeneron Pharmaceuticals, Inc.
For In Vitro Diagnostic Use.